Filter News
Area of Research
News Topics
- (-) Critical Materials (26)
- (-) Isotopes (53)
- (-) Molten Salt (8)
- 3-D Printing/Advanced Manufacturing (121)
- Advanced Reactors (34)
- Artificial Intelligence (91)
- Big Data (53)
- Bioenergy (91)
- Biology (98)
- Biomedical (58)
- Biotechnology (22)
- Buildings (57)
- Chemical Sciences (63)
- Clean Water (29)
- Climate Change (99)
- Composites (26)
- Computer Science (187)
- Coronavirus (46)
- Cybersecurity (35)
- Decarbonization (79)
- Education (4)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (108)
- Environment (194)
- Exascale Computing (37)
- Fossil Energy (5)
- Frontier (42)
- Fusion (54)
- Grid (62)
- High-Performance Computing (84)
- Hydropower (11)
- Irradiation (3)
- ITER (7)
- Machine Learning (47)
- Materials (144)
- Materials Science (140)
- Mathematics (7)
- Mercury (12)
- Microelectronics (3)
- Microscopy (51)
- Nanotechnology (60)
- National Security (61)
- Net Zero (13)
- Neutron Science (131)
- Nuclear Energy (108)
- Partnerships (44)
- Physics (61)
- Polymers (33)
- Quantum Computing (34)
- Quantum Science (69)
- Renewable Energy (2)
- Security (24)
- Simulation (47)
- Software (1)
- Space Exploration (25)
- Statistics (3)
- Summit (57)
- Sustainable Energy (125)
- Transformational Challenge Reactor (7)
- Transportation (97)
Media Contacts

East Tennessee occupies a special place in nuclear history. In 1943, the world’s first continuously operating reactor began operating on land that would become ORNL.

ORNL has added 10 virtual tours to its campus map, each with multiple views to show floor plans, rotating dollhouse views and 360-degree navigation. As a user travels through a map, pop-out informational windows deliver facts, videos, graphics and links to other related content.

Momentum Technologies Inc., a Dallas, Texas-based materials science company that is focused on extracting critical metals from electronic waste, has licensed an Oak Ridge National Laboratory process for recovering cobalt and other metals from spent

Radioactive isotopes power some of NASA’s best-known spacecraft. But predicting how radiation emitted from these isotopes might affect nearby materials is tricky
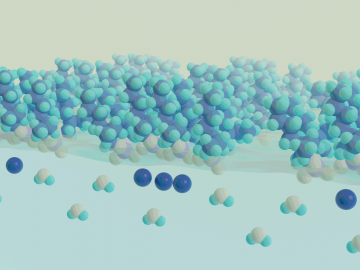
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.

After its long journey to Mars beginning this summer, NASA’s Perseverance rover will be powered across the planet’s surface in part by plutonium produced at the Department of Energy’s Oak Ridge National Laboratory.

Oak Ridge National Laboratory researchers have discovered a better way to separate actinium-227, a rare isotope essential for an FDA-approved cancer treatment.

In the 1960s, Oak Ridge National Laboratory's four-year Molten Salt Reactor Experiment tested the viability of liquid fuel reactors for commercial power generation. Results from that historic experiment recently became the basis for the first-ever molten salt reactor benchmark.

Oak Ridge National Laboratory scientists analyzed more than 50 years of data showing puzzlingly inconsistent trends about corrosion of structural alloys in molten salts and found one factor mattered most—salt purity.

OAK RIDGE, Tenn., Jan. 31, 2019—A new electron microscopy technique that detects the subtle changes in the weight of proteins at the nanoscale—while keeping the sample intact—could open a new pathway for deeper, more comprehensive studies of the basic building blocks of life.


