
Filter News
Area of Research
- Biology and Environment (28)
- Biology and Soft Matter (1)
- Clean Energy (46)
- Climate and Environmental Systems (1)
- Electricity and Smart Grid (1)
- Functional Materials for Energy (1)
- Fusion and Fission (3)
- Materials (23)
- Materials for Computing (1)
- National Security (2)
- Neutron Science (2)
- Nuclear Science and Technology (5)
- Supercomputing (9)
- Transportation Systems (1)
News Topics
- (-) Critical Materials (26)
- (-) Decarbonization (80)
- (-) Mercury (12)
- (-) Molten Salt (8)
- 3-D Printing/Advanced Manufacturing (122)
- Advanced Reactors (34)
- Artificial Intelligence (91)
- Big Data (55)
- Bioenergy (92)
- Biology (99)
- Biomedical (58)
- Biotechnology (22)
- Buildings (57)
- Chemical Sciences (65)
- Clean Water (29)
- Climate Change (100)
- Composites (26)
- Computer Science (189)
- Coronavirus (46)
- Cybersecurity (35)
- Education (4)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (109)
- Environment (195)
- Exascale Computing (37)
- Fossil Energy (6)
- Frontier (42)
- Fusion (55)
- Grid (63)
- High-Performance Computing (85)
- Hydropower (11)
- Irradiation (3)
- Isotopes (53)
- ITER (7)
- Machine Learning (48)
- Materials (144)
- Materials Science (141)
- Mathematics (8)
- Microelectronics (3)
- Microscopy (51)
- Nanotechnology (60)
- National Security (62)
- Net Zero (14)
- Neutron Science (131)
- Nuclear Energy (109)
- Partnerships (44)
- Physics (61)
- Polymers (33)
- Quantum Computing (34)
- Quantum Science (69)
- Renewable Energy (2)
- Security (24)
- Simulation (48)
- Software (1)
- Space Exploration (25)
- Statistics (3)
- Summit (57)
- Sustainable Energy (126)
- Transformational Challenge Reactor (7)
- Transportation (97)
Media Contacts

Scientists at ORNL have discovered a single gene that simultaneously boosts plant growth and tolerance for stresses such as drought and salt, all while tackling the root cause of climate change by enabling plants to pull more carbon dioxide from the atmosphere.
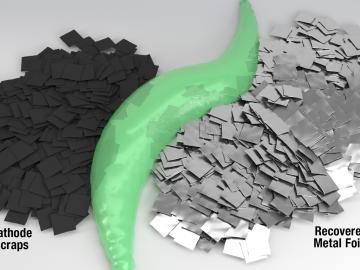
Scientists at Oak Ridge National Laboratory have developed a solvent that results in a more environmentally friendly process to recover valuable materials from used lithium-ion batteries, supports a stable domestic supply chain for new batteries
Scientists at Oak Ridge National Laboratory have devised a method to identify the unique chemical makeup of every lithium-ion battery around the world, information that could accelerate recycling, recover critical materials and resolve a growing waste stream.

When Kashif Nawaz looks at a satellite map of the U.S., he sees millions of buildings that could hold a potential solution for the capture of carbon dioxide, a plentiful gas that can be harmful when excessive amounts are released into the atmosphere, raising the Earth’s temperature.

Six scientists at the Department of Energy’s Oak Ridge National Laboratory were named Battelle Distinguished Inventors, in recognition of obtaining 14 or more patents during their careers at the lab.

New capabilities and equipment recently installed at the Department of Energy’s Oak Ridge National Laboratory are bringing a creek right into the lab to advance understanding of mercury pollution and accelerate solutions.

Momentum Technologies Inc., a Dallas, Texas-based materials science company that is focused on extracting critical metals from electronic waste, has licensed an Oak Ridge National Laboratory process for recovering cobalt and other metals from spent
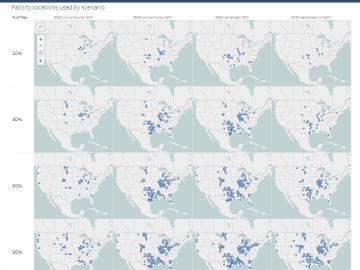
The combination of bioenergy with carbon capture and storage could cost-effectively sequester hundreds of millions of metric tons per year of carbon dioxide in the United States, making it a competitive solution for carbon management, according to a new analysis by ORNL scientists.
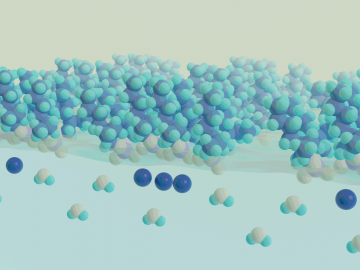
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.

In the 1960s, Oak Ridge National Laboratory's four-year Molten Salt Reactor Experiment tested the viability of liquid fuel reactors for commercial power generation. Results from that historic experiment recently became the basis for the first-ever molten salt reactor benchmark.


