Filter News
Area of Research
News Topics
- (-) Molten Salt (8)
- (-) Polymers (31)
- 3-D Printing/Advanced Manufacturing (116)
- Advanced Reactors (33)
- Artificial Intelligence (84)
- Big Data (50)
- Bioenergy (88)
- Biology (96)
- Biomedical (58)
- Biotechnology (21)
- Buildings (54)
- Chemical Sciences (59)
- Clean Water (29)
- Climate Change (94)
- Composites (25)
- Computer Science (182)
- Coronavirus (46)
- Critical Materials (24)
- Cybersecurity (35)
- Decarbonization (73)
- Education (3)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (106)
- Environment (192)
- Exascale Computing (34)
- Fossil Energy (5)
- Frontier (39)
- Fusion (53)
- Grid (59)
- High-Performance Computing (82)
- Hydropower (11)
- Irradiation (3)
- Isotopes (47)
- ITER (7)
- Machine Learning (44)
- Materials (140)
- Materials Science (134)
- Mathematics (6)
- Mercury (12)
- Microelectronics (2)
- Microscopy (50)
- Nanotechnology (60)
- National Security (57)
- Net Zero (11)
- Neutron Science (129)
- Nuclear Energy (105)
- Partnerships (40)
- Physics (58)
- Quantum Computing (29)
- Quantum Science (65)
- Renewable Energy (2)
- Security (24)
- Simulation (43)
- Software (1)
- Space Exploration (24)
- Statistics (3)
- Summit (57)
- Sustainable Energy (119)
- Transformational Challenge Reactor (7)
- Transportation (93)
Media Contacts

ORNL and Department of Energy officials dedicated the launch of two clean energy research initiatives that focus on the recycling and recovery of advanced manufacturing materials and on connected and
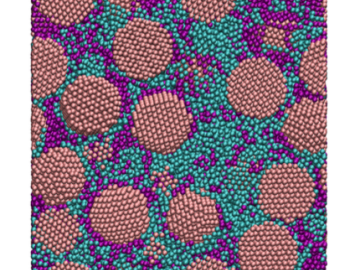
Oak Ridge National Laboratory scientists have discovered a cost-effective way to significantly improve the mechanical performance of common polymer nanocomposite materials.
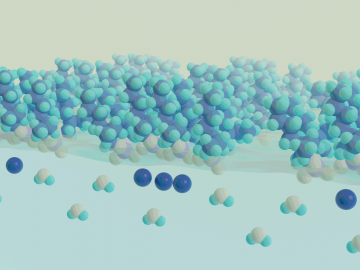
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.
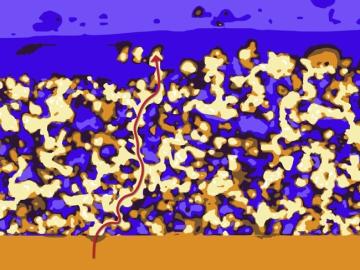
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.

In the 1960s, Oak Ridge National Laboratory's four-year Molten Salt Reactor Experiment tested the viability of liquid fuel reactors for commercial power generation. Results from that historic experiment recently became the basis for the first-ever molten salt reactor benchmark.
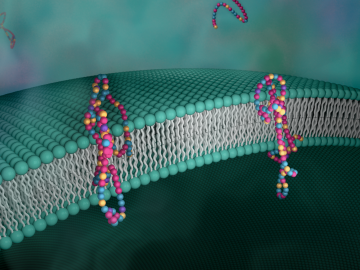
Biological membranes, such as the “walls” of most types of living cells, primarily consist of a double layer of lipids, or “lipid bilayer,” that forms the structure, and a variety of embedded and attached proteins with highly specialized functions, including proteins that rapidly and selectively transport ions and molecules in and out of the cell.
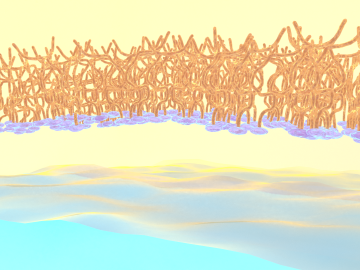
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.
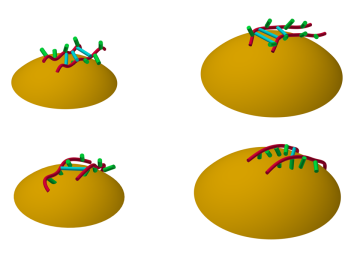
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

Oak Ridge National Laboratory scientists analyzed more than 50 years of data showing puzzlingly inconsistent trends about corrosion of structural alloys in molten salts and found one factor mattered most—salt purity.