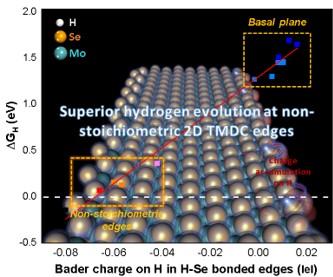
The Figure shows the linear correlation of charges on H and ∆GH in the H-Se bonded edges. The yellow boxes are results for non-stoichiometric edges and the basal plane and the white dash line indicates ∆GH of zero.
Scientific Achievement
Non-stoichiometric edges in 2D transition metal dichalcogenides (TMDs) were created and demonstrated to have near optimal hydrogen evolution reaction (HER) activity (i.e., Gibbs free energy, ∆GH=0).
Significance and Impact
Compared to the stoichiometric TMD edges, there is a wider family of synthesizable non-stoichiometric TMD edges, where the degree of non-stoichiometry can be tuned to dial-in optimal HER activity.


