Filter News
Area of Research
News Topics
- (-) Artificial Intelligence (1)
- (-) Energy Storage (9)
- 3-D Printing/Advanced Manufacturing (9)
- Advanced Reactors (2)
- Bioenergy (3)
- Biomedical (1)
- Buildings (3)
- Chemical Sciences (16)
- Clean Water (2)
- Climate Change (3)
- Composites (3)
- Computer Science (4)
- Coronavirus (1)
- Critical Materials (4)
- Cybersecurity (1)
- Decarbonization (4)
- Environment (8)
- Fusion (4)
- Grid (2)
- Irradiation (1)
- Isotopes (4)
- Materials (40)
- Materials Science (25)
- Microscopy (8)
- Molten Salt (2)
- Nanotechnology (9)
- Net Zero (1)
- Neutron Science (12)
- Nuclear Energy (10)
- Partnerships (6)
- Physics (11)
- Polymers (6)
- Quantum Computing (2)
- Quantum Science (3)
- Renewable Energy (1)
- Sustainable Energy (6)
- Transportation (7)
Media Contacts

In fiscal year 2023 — Oct. 1–Sept. 30, 2023 — Oak Ridge National Laboratory was awarded more than $8 million in technology maturation funding through the Department of Energy’s Technology Commercialization Fund, or TCF.
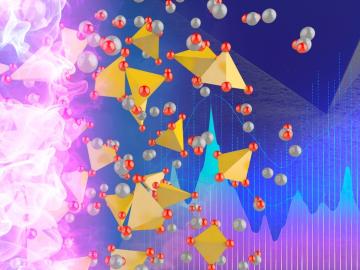
As current courses through a battery, its materials erode over time. Mechanical influences such as stress and strain affect this trajectory, although their impacts on battery efficacy and longevity are not fully understood.

ORNL has been selected to lead an Energy Earthshot Research Center, or EERC, focused on developing chemical processes that use sustainable methods instead of burning fossil fuels to radically reduce industrial greenhouse gas emissions to stem climate change and limit the crisis of a rapidly warming planet.
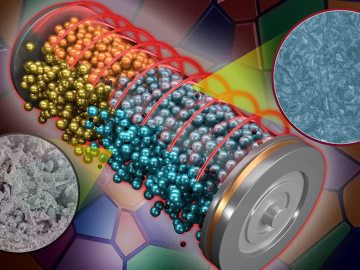
ORNL scientists found that a small tweak created big performance improvements in a type of solid-state battery, a technology considered vital to broader electric vehicle adoption.

Andrew Ullman, Distinguished Staff Fellow at Oak Ridge National Laboratory, is using chemistry to devise a better battery

Students often participate in internships and receive formal training in their chosen career fields during college, but some pursue professional development opportunities even earlier.
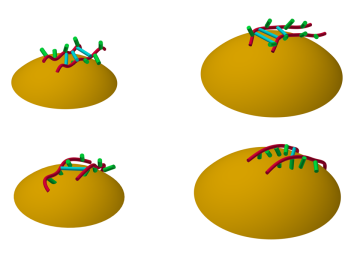
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.
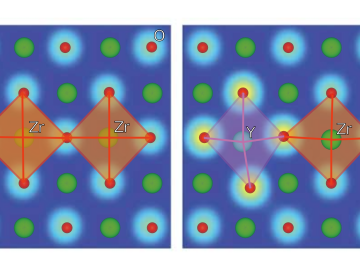
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
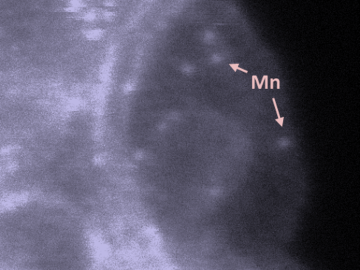
Oak Ridge National Laboratory scientists studying fuel cells as a potential alternative to internal combustion engines used sophisticated electron microscopy to investigate the benefits of replacing high-cost platinum with a lower cost, carbon-nitrogen-manganese-based catalyst.




