
Filter News
Area of Research
- (-) Fusion Energy (8)
- (-) Materials (75)
- (-) National Security (44)
- Advanced Manufacturing (5)
- Biology and Environment (36)
- Building Technologies (1)
- Clean Energy (57)
- Climate and Environmental Systems (1)
- Computational Biology (1)
- Computational Engineering (3)
- Computer Science (15)
- Energy Frontier Research Centers (1)
- Fusion and Fission (11)
- Isotopes (1)
- Materials for Computing (15)
- Mathematics (1)
- Neutron Science (105)
- Nuclear Science and Technology (14)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Quantum information Science (6)
- Sensors and Controls (1)
- Supercomputing (115)
News Type
News Topics
- (-) Advanced Reactors (10)
- (-) Artificial Intelligence (20)
- (-) Computer Science (35)
- (-) Cybersecurity (20)
- (-) Machine Learning (15)
- (-) Nanotechnology (37)
- (-) Neutron Science (33)
- (-) Security (11)
- 3-D Printing/Advanced Manufacturing (25)
- Big Data (7)
- Bioenergy (14)
- Biology (8)
- Biomedical (8)
- Biotechnology (1)
- Buildings (5)
- Chemical Sciences (31)
- Clean Water (3)
- Climate Change (9)
- Composites (9)
- Coronavirus (6)
- Critical Materials (13)
- Decarbonization (8)
- Energy Storage (33)
- Environment (19)
- Exascale Computing (2)
- Frontier (3)
- Fusion (16)
- Grid (10)
- High-Performance Computing (7)
- Isotopes (13)
- ITER (1)
- Materials (70)
- Materials Science (71)
- Mathematics (1)
- Microscopy (24)
- Molten Salt (3)
- National Security (33)
- Net Zero (1)
- Nuclear Energy (25)
- Partnerships (15)
- Physics (27)
- Polymers (16)
- Quantum Computing (3)
- Quantum Science (12)
- Renewable Energy (1)
- Simulation (1)
- Space Exploration (2)
- Summit (5)
- Sustainable Energy (16)
- Transformational Challenge Reactor (3)
- Transportation (16)
Media Contacts
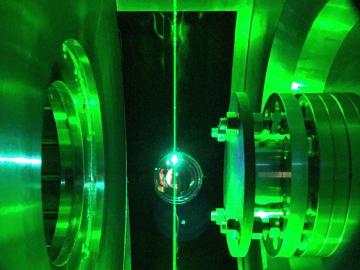
In a recent study, researchers at Oak Ridge National Laboratory performed experiments in a prototype fusion reactor materials testing facility to develop a method that uses microwaves to raise the plasma’s temperature closer to the extreme values

IDEMIA Identity & Security USA has licensed an advanced optical array developed at Oak Ridge National Laboratory. The portable technology can be used to help identify individuals in challenging outdoor conditions.

Using additive manufacturing, scientists experimenting with tungsten at Oak Ridge National Laboratory hope to unlock new potential of the high-performance heat-transferring material used to protect components from the plasma inside a fusion reactor. Fusion requires hydrogen isotopes to reach millions of degrees.
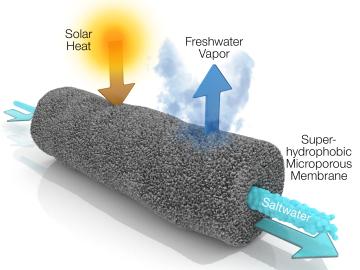
A new method developed at Oak Ridge National Laboratory improves the energy efficiency of a desalination process known as solar-thermal evaporation.
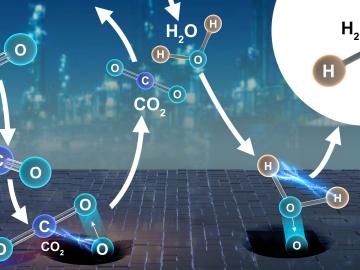
Collaborators at the Department of Energy’s Oak Ridge National Laboratory and U.S. universities used neutron scattering and other advanced characterization techniques to study how a prominent catalyst enables the “water-gas shift” reaction to purify and generate hydrogen at industrial scale.

Researchers have pioneered a new technique using pressure to manipulate magnetism in thin film materials used to enhance performance in electronic devices.
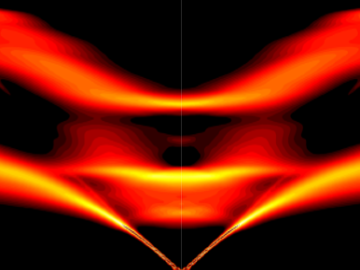
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials
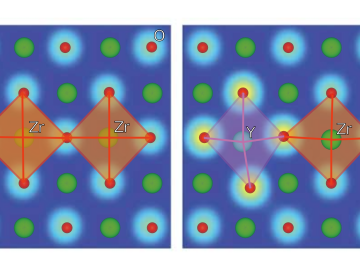
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.

Scientists at the Department of Energy’s Oak Ridge National Laboratory are working to understand both the complex nature of uranium and the various oxide forms it can take during processing steps that might occur throughout the nuclear fuel cycle.
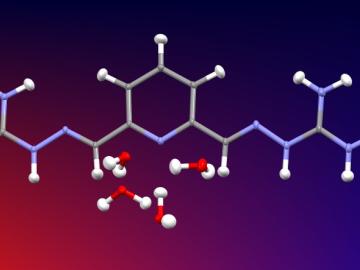
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.


