
Filter News
Area of Research
- (-) Clean Energy (73)
- (-) Neutron Science (48)
- Advanced Manufacturing (8)
- Biological Systems (2)
- Biology and Environment (78)
- Computational Biology (2)
- Computational Engineering (2)
- Computer Science (2)
- Electricity and Smart Grid (1)
- Functional Materials for Energy (1)
- Fusion and Fission (7)
- Fusion Energy (3)
- Isotope Development and Production (1)
- Isotopes (7)
- Materials (124)
- Materials Characterization (1)
- Materials for Computing (18)
- Materials Under Extremes (1)
- Mathematics (1)
- National Security (11)
- Nuclear Science and Technology (12)
- Quantum information Science (2)
- Supercomputing (76)
- Transportation Systems (1)
News Topics
- (-) Bioenergy (30)
- (-) Biomedical (17)
- (-) Clean Water (10)
- (-) Composites (18)
- (-) Materials Science (48)
- (-) Molten Salt (1)
- (-) Physics (10)
- (-) Summit (9)
- 3-D Printing/Advanced Manufacturing (82)
- Advanced Reactors (6)
- Artificial Intelligence (14)
- Big Data (7)
- Biology (16)
- Biotechnology (5)
- Buildings (36)
- Chemical Sciences (16)
- Climate Change (22)
- Computer Science (35)
- Coronavirus (20)
- Critical Materials (9)
- Cybersecurity (9)
- Decarbonization (34)
- Energy Storage (75)
- Environment (59)
- Exascale Computing (2)
- Fossil Energy (3)
- Frontier (3)
- Fusion (2)
- Grid (40)
- High-Performance Computing (8)
- Hydropower (2)
- Isotopes (1)
- Machine Learning (10)
- Materials (46)
- Mathematics (3)
- Mercury (3)
- Microelectronics (1)
- Microscopy (10)
- Nanotechnology (17)
- National Security (7)
- Net Zero (3)
- Neutron Science (100)
- Nuclear Energy (9)
- Partnerships (12)
- Polymers (12)
- Quantum Computing (1)
- Quantum Science (8)
- Renewable Energy (1)
- Security (8)
- Simulation (4)
- Space Exploration (6)
- Statistics (1)
- Sustainable Energy (69)
- Transformational Challenge Reactor (3)
- Transportation (67)
Media Contacts
Research by an international team led by Duke University and the Department of Energy’s Oak Ridge National Laboratory scientists could speed the way to safer rechargeable batteries for consumer electronics such as laptops and cellphones.
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
In the race to identify solutions to the COVID-19 pandemic, researchers at the Department of Energy’s Oak Ridge National Laboratory are joining the fight by applying expertise in computational science, advanced manufacturing, data science and neutron science.
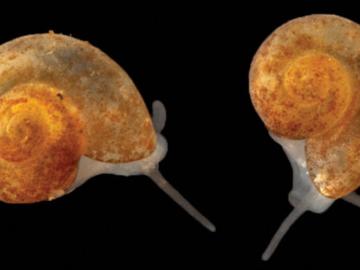
Sometimes conducting big science means discovering a species not much larger than a grain of sand.
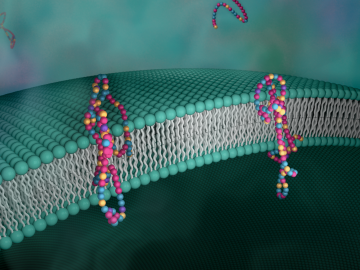
Biological membranes, such as the “walls” of most types of living cells, primarily consist of a double layer of lipids, or “lipid bilayer,” that forms the structure, and a variety of embedded and attached proteins with highly specialized functions, including proteins that rapidly and selectively transport ions and molecules in and out of the cell.
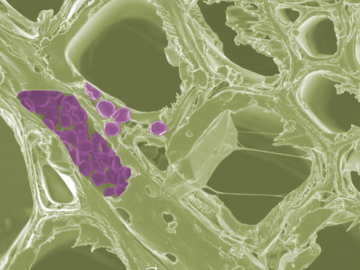
Scientists at the Department of Energy’s Oak Ridge National Laboratory have developed a new method to peer deep into the nanostructure of biomaterials without damaging the sample. This novel technique can confirm structural features in starch, a carbohydrate important in biofuel production.
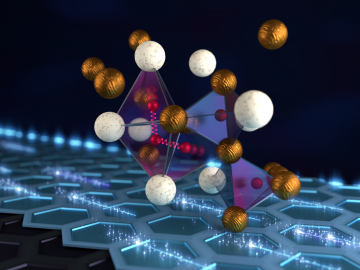
An international team of researchers has discovered the hydrogen atoms in a metal hydride material are much more tightly spaced than had been predicted for decades — a feature that could possibly facilitate superconductivity at or near room temperature and pressure.

The formation of lithium dendrites is still a mystery, but materials engineers study the conditions that enable dendrites and how to stop them.
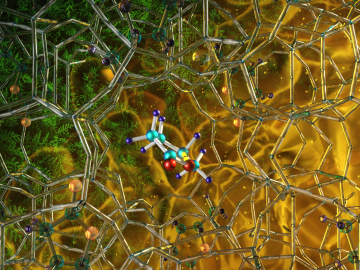
Illustration of the optimized zeolite catalyst, or NbAlS-1, which enables a highly efficient chemical reaction to create butene, a renewable source of energy, without expending high amounts of energy for the conversion. Credit: Jill Hemman, Oak Ridge National Laboratory/U.S. Dept. of Energy

While Tsouris’ water research is diverse in scope, its fundamentals are based on basic science principles that remain largely unchanged, particularly in a mature field like chemical engineering.


