Filter News
Area of Research
- (-) Materials Synthesis from Atoms to Systems (13)
- (-) Neutron Science (190)
- Advanced Manufacturing (34)
- Biological Systems (18)
- Biology and Environment (177)
- Biology and Soft Matter (5)
- Building Technologies (12)
- Chemical and Engineering Materials (4)
- Chemistry and Physics at Interfaces (11)
- Clean Energy (522)
- Climate and Environmental Systems (14)
- Computational Biology (6)
- Computational Chemistry (5)
- Computational Engineering (5)
- Computer Science (19)
- Data (1)
- Earth Sciences (1)
- Electricity and Smart Grid (3)
- Energy Frontier Research Centers (14)
- Energy Sciences (5)
- Fossil Energy (3)
- Fuel Cycle Science and Technology (3)
- Functional Materials for Energy (16)
- Fusion and Fission (54)
- Fusion Energy (17)
- Geographic Information Science and Technology (3)
- Isotope Development and Production (3)
- Isotopes (35)
- Materials (433)
- Materials Characterization (2)
- Materials for Computing (36)
- Materials Under Extremes (12)
- Mathematics (1)
- National Security (79)
- Neutron Data Analysis and Visualization (4)
- Nuclear Science and Technology (74)
- Nuclear Systems Modeling, Simulation and Validation (3)
- Nuclear Systems Technology (1)
- Quantum Condensed Matter (4)
- Quantum information Science (9)
- Reactor Technology (1)
- Renewable Energy (4)
- Sensors and Controls (5)
- Supercomputing (311)
- Transportation Systems (11)
News Type
News Topics
- 3-D Printing/Advanced Manufacturing (6)
- Advanced Reactors (1)
- Artificial Intelligence (6)
- Big Data (2)
- Bioenergy (6)
- Biology (5)
- Biomedical (11)
- Biotechnology (1)
- Chemical Sciences (2)
- Clean Water (2)
- Climate Change (1)
- Composites (1)
- Computer Science (13)
- Coronavirus (8)
- Cybersecurity (1)
- Decarbonization (2)
- Energy Storage (6)
- Environment (8)
- Fossil Energy (1)
- Frontier (1)
- Fusion (1)
- High-Performance Computing (2)
- Machine Learning (3)
- Materials (14)
- Materials Science (23)
- Mathematics (1)
- Microscopy (3)
- Nanotechnology (10)
- National Security (2)
- Neutron Science (99)
- Nuclear Energy (3)
- Physics (9)
- Polymers (1)
- Quantum Computing (1)
- Quantum Science (7)
- Security (2)
- Space Exploration (3)
- Summit (6)
- Sustainable Energy (2)
- Transportation (5)
Media Contacts



From the bluebird painting propped against her office wall and the deer she mentions seeing outside her office window, Linda Lewis might be mistaken for a wildlife biologist at first glance. But rather than trailing animal tracks, Lewis, a researcher at the Department of Energy’s Oak Ridge National Laboratory, is more interested in marks left behind by humans.

With more than 30 patents, James Klett is no stranger to success, but perhaps the Oak Ridge National Laboratory researcher’s most noteworthy achievement didn’t start out so hot – or so it seemed at the time.

Graphene, a strong, lightweight carbon honeycombed structure that’s only one atom thick, holds great promise for energy research and development. Recently scientists with the Fluid Interface Reactions, Structures, and Transport (FIRST) Energy Frontier Research Center (EFRC), led by the US Department of Energy’s Oak Ridge National Laboratory, revealed graphene can serve as a proton-selective permeable membrane, providing a new basis for streamlined and more efficient energy technologies such as improved fuel cells.
Andrew Stack, a geochemist at the Department of Energy’s Oak Ridge National Laboratory, advances understanding of the dynamics of minerals underground.
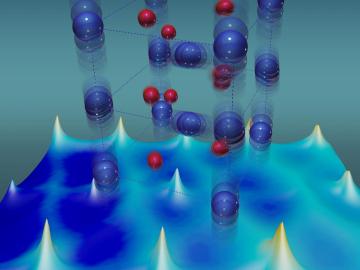
For more than 50 years, scientists have debated what turns particular oxide insulators, in which electrons barely move, into metals, in which electrons flow freely.